Introduction#
When you do an electrochemical experiment, the composition of your electrode can change depending on the conditions. For example, a metal electrode can oxidize, forming a metal oxide. Under different conditions, the metal can dissolve. The two main parameters affecting stability in a water-based electrolyte are electrode potential and pH. A Pourbaix diagram visualizes which material phase is stable under which conditions.
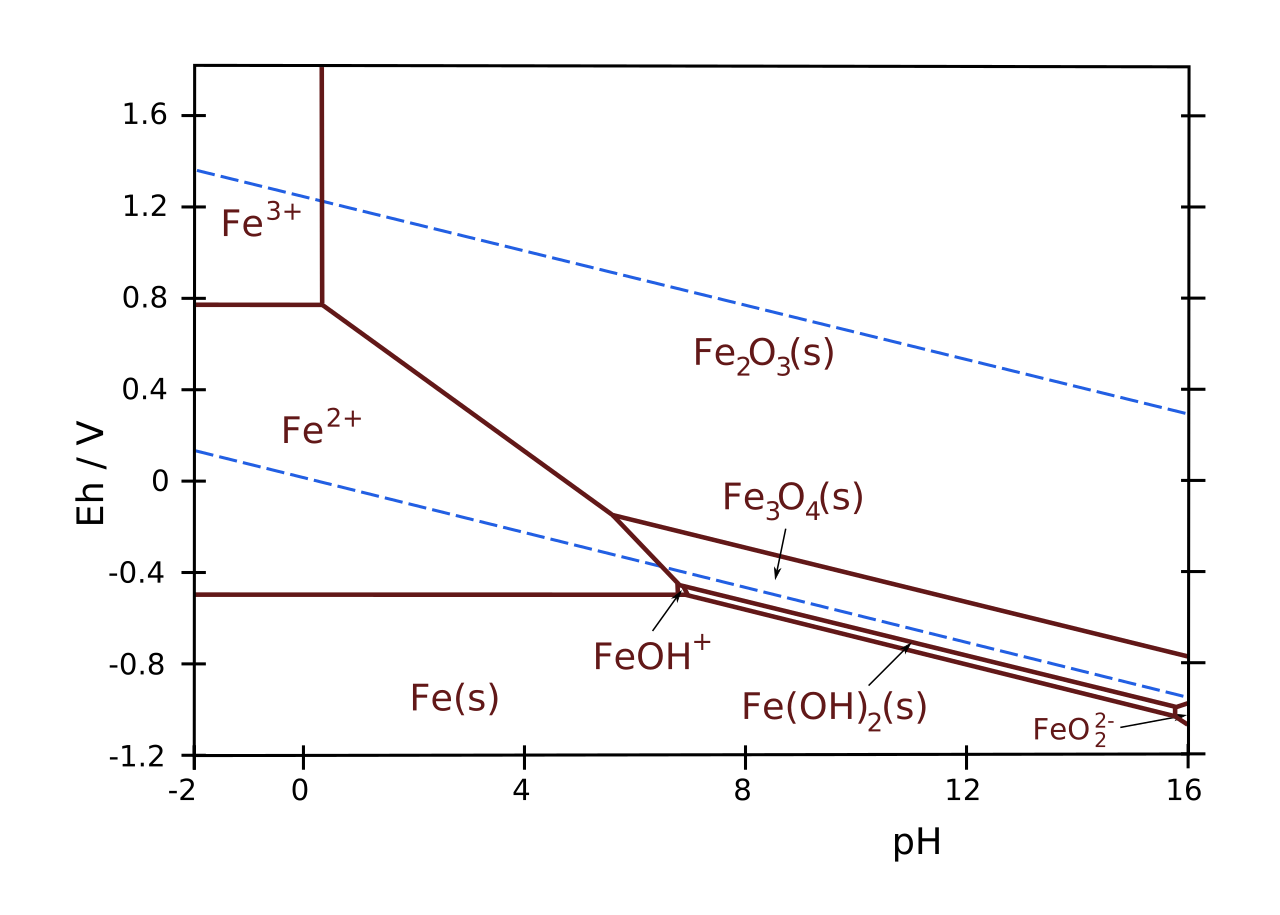
Water stability#
The dashed blue lines in the Pourbaix diagram above indicate the water stability window. If you go below the lower line, you produce hydrogen according to the reaction
This reaction is called the hydrogen evolution reaction (HER). Above the upper line, the oxygen evolution reaction (OER)
occurs. Typically, an experiment is done within the water stability window to avoid the formation of gas bubbles.
Questions
What iron oxide phases can you encounter in a typical electrochemical experiment?
What do the HER and OER look like in alkaline media?
Reference points and SHE#
Throughout this lab course, the concept of a ‘reference point’ will return several times. We cannot measure an absolute value for electric potentials and energies; we always measure the difference with respect to some reference point.
In electrochemistry, the most common reference point for the electrode potential is the standard hydrogen electrode (SHE). It’s defined as a platinum electrode where the hydrogen evolution reaction (1) occurs under standard conditions (1 bar \(\mathrm{H_2}\)-pressure, 1 M \(\mathrm{H^+}\)-concentration, i.e. pH 0). When using the SHE as a reference, the equilibrium potential of the SHE is defined as zero.
Questions
What point in the Pourbaix diagram corresponds to the SHE?
In experiments, we often use a different reference electrode: the reversible hydrogen electrode (RHE). It’s almost the same as SHE, except that the \(\mathrm{H^+}\)-concentration is allowed to change, depending on your experiment. The zero on the RHE scale corresponds to the blue dotted line in the Pourbaix diagram Pourbaix diagram of iron (Wikipedia)..