Porphyrins#
Porphyrins are complexes based on a porphine molecule bound to a metal ion in the center. They are found in various biological systems. For example, heme is a porphyrin, which is a part of the hemoglobin complex that binds oxygen in your blood. Chlorophyll, which is responsible for photosynthesis, also has a porphyrin group.

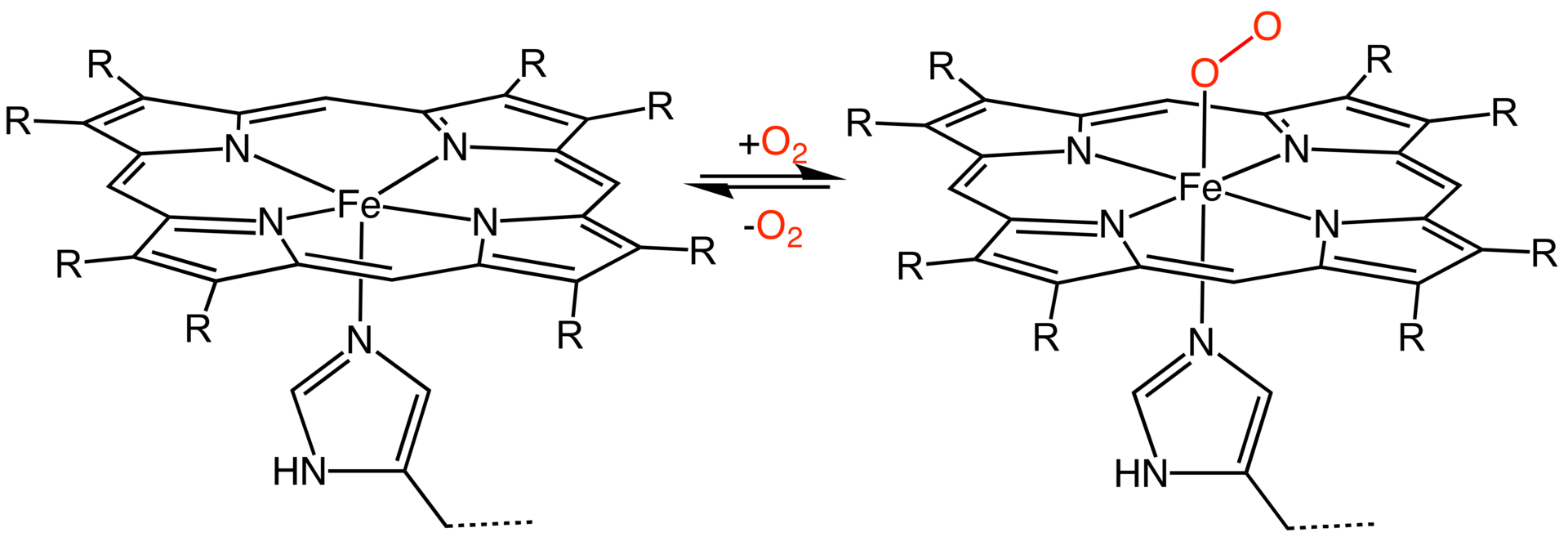
In the electrochemistry project you will do or have done, porphyrins are deposited on a surface. Their metal centers can catalyze the oxygen reduction reaction. In this project, we will use density functional theory calculations to calculate the binding energy of the oxygen molecule.
Density functional theory for molecules#
Remember that our goal is to find the ground-state energy of various molecules, from which we can then calculate the binding energy.
To this end we want to solve the Kohn-Sham equations for the orbitals (one-electron wave functions) \(\psi_i\) and their energies \(\varepsilon_i\). Wave functions are functions, which you could view as an infinite collection of numbers: there are infinitely many points in space, and each space has a corresponding value of the wavefunction. How do we put this into a computer?
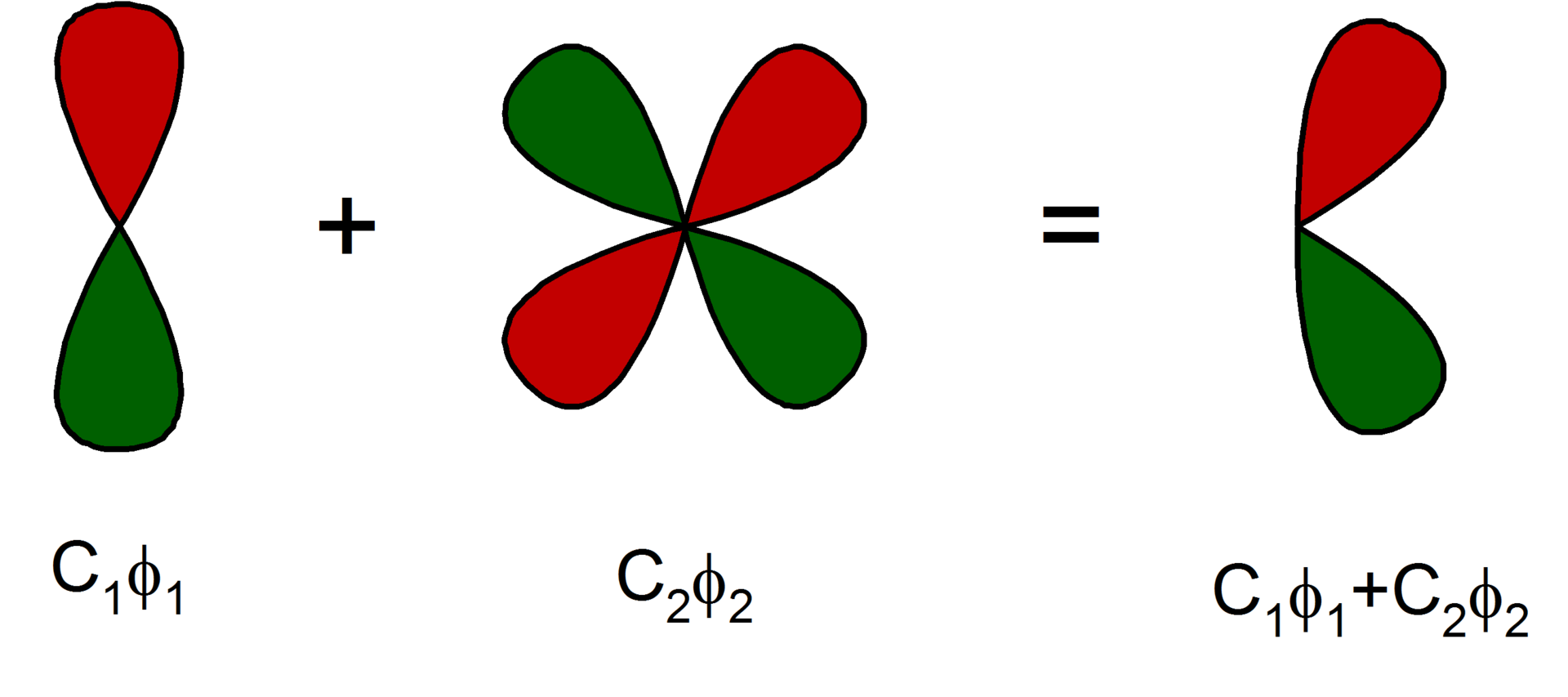
One option would be to divide space into a grid (like a 3D chess board) and solve the wave function in each cube. It turns out that this is not very efficient. Instead, it turns out that wave functions usually have pretty similar shapes. In practice we ‘build’ each orbital out of a small collection of basic functions \(\phi_i\):
The \(\phi_i\) are called basis functions, and \(c_i\) are the coefficients that scale them. The collection of all \(\phi_i\) is called a basis set. The image below shows an example.
Now the computer just needs to calculate the coefficients \(c_i\). For the radial part, one popular basis function is an exponential function
Different basis functions are have different values of \(\zeta\). The symbol \(\zeta\) is called a zeta, so a collection of three basis functions is often called a triple-zeta basis set (TZ), for example. The more basis functions you choose, the closer you can get to the ground state energy. However, the calculation might take longer. You can read more about basis sets here (not required).
In the following, we will use the Amsterdam Modelling Suite (AMS) software to calculate the binding energy of oxygen to porphyrin molecules. The software is available here: https://www.scm.com/support/downloads/. Ask your supervisor for instructions to install with the university license.
First, let’s get used to the software.
Task 1: geometry optimization of ethanol#
Follow the tutorial on the website of AMS SCM. Pay attention to the following things.
In the
.logfile: look for the word SCF, which stands for ‘self consistent field’. You can see that the software is running multiple cycles of the self-consistent procedure.Notice that the energy is minimized until it does not change much anymore. The change in energy before and after solving the Kohn-Sham equations is denoted as absolute error (
|Error|).Practice saving images, which you will need later for writing your report (you don’t need to write about the ethanol tutorial, though).
Now we are ready to do our first ‘useful’ calculation.
Task 2: simulate an oxygen molecule#
In a new AMS input file, build an oxygen molecule. Pre-optimize the geometry with a simplified calculation method (force fields) using the button:

The ground state of oxygen has two unpaired electrons. Using the internet or your own knowledge, find out how many electrons are unpaired. The number of unpaired electrons should be entered into the field ‘Spin polarization’. To enter something in this field, you need to check the ‘Unrestricted’ box.
In the rest of the practical we will use the XC functional BP86 (find it under the GGA group) and a TZ2P basis set. Choose the same settings here for consistency. (Convince yourself that this is important.)
Write down the energy you get. How to find the energy? Have a look at this page
Change the task from ‘Single Point’ to ‘Geometry Optimization’ and check the ‘Frequencies’ box so that we optimize the geometry and obtain information about the vibrations of an oxygen molecule.
The vibrational frequency is in the
.outfile. Compare it to a value you find from a (reliable) source on the internet.Save the thermodynamic information (Statistical Thermal Analysis at the end of the
.outfile.).
Task 3: simulate a metalloporphyrin#
First, write down which metal ion is in the porphyrin you have researched or will research in the lab part of LO1. This should be iron for Team 3, cobalt for Team 5, and any of iron, cobalt and manganese for Team 4. If we don’t have much time, choose iron.
In a new AMS input file, create a porphyrin molecule using the following shortcut:
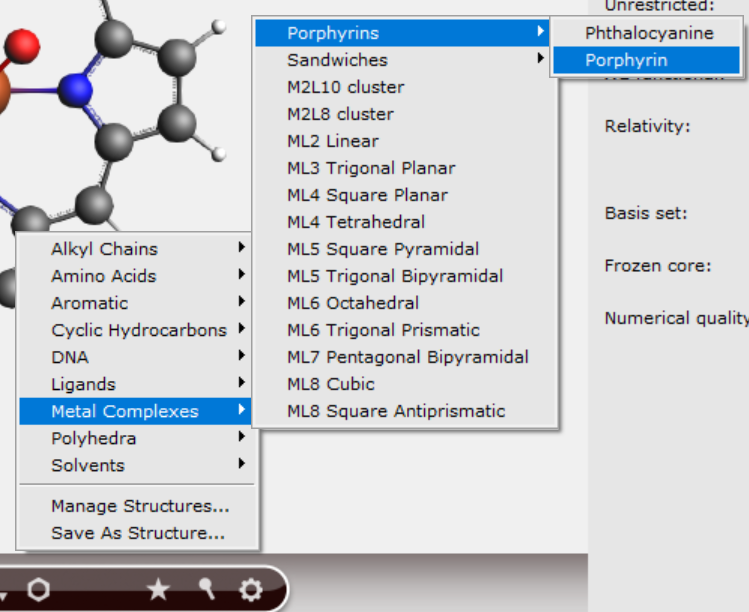
Remove the two ‘attachment’ sticks on the side of the iron center.
Replace the metal center with the relevant metal atom, and pre-optimize the geometry.
The electronic configuration of the metals is something like \(\mathrm{[Ar]} 3d^n 4s^2\), where \(n\) depends on the metal. The metal center usually has oxidation state +2, so Fe(II) [2], Mn(II) [3], or Co(II) [1, 4]. The two electrons are removed from \(4s\). The \(3d\) orbitals (which can hold max. 10 electrons) are partially filled.
Write down the different possible spin configurations. What is the number of unpaired electrons for each of them?
Using the following articles, find what the ground state of your porphyrin is. Enter the number of unpaired electrons in the field ‘Spin polarization’ (check ‘Unrestricted’ as ‘Yes’).
We use the GGA XC functional BP86 as recommended by Ovalle and Malardier-Jugroot [8] with a TZ2P basis set (should be more or less similar to their basis set A). Use a ‘large’ frozen core and a ‘scalar’ relativity correction.
Calculate and note down the energy.
Task 4: simulate a metalloporphyrin binding an oxygen molecule#
Oxygen can bind to the metal center. In this bond, the valence orbitals of oxygen and the metal interact. When looking at the spin state of oxygen before, you found that some electrons are unpaired.
Using Fig. 3 in Phung and Pierloot [3], construct molecular orbital diagrams (there may be multiple possibilities) for a metalloporphyrin bonded to \(\mathrm{O_2}\).
Based on Phung and Pierloot [3], Rovira and Parrinello [6] we’ll assume that the ground-state of the oxygen-bound complex usually has the lowest possible spin.
How many unpaired electrons are there in the final molecule?
Create a new AMS input file. Add a porphyrin and add an \(\mathrm{O_2}\) molecule that binds with one atom to the metal center. Pre-optimize the geometry.
Compare your pre-optimized geometry to figures in Rovira and Parrinello [6]. What can you conclude about the accuracy of the energy calculation, if we only pre-optimize the structure like we have now?
Calculate the energy with the same settings as in the previous exercise. Note down the energy.
If you go to SCM -> Levels, right-click on one of the molecular orbitals and select the first option, you can visualize the orbitals. Visualize the HOMO and compare to Fig. 3 in Phung and Pierloot [3].
We don’t have time to do frequency calculations. Note down BP86 thermal corrections from literature. Tip: convert everything to the same unit, like eV
Task 5: binding energy#
Now we have all the necessary ingredients to calculate the binding energy.
Calculate the Gibbs binding energy. Compare to Table 2 in Phung and Pierloot [3] and Table 6 in Radon and Pierloot [2] (five-coordinated complexes).
Why could there be differences? Try to find out using [3], for example. Note: DMRG-CASPT2 is a very accurate wave function method, that does not suffer from many of the approximations made in DFT. Also think about the spin states we assumed, and our geometry optimization.
Task 6: small research project#
Choose an interesting research project, and do your own calculations. Compare the results to literature if you can. Here’s some ideas:
Vary the metal center of the porphyrin (suggested for Team 4).
Compare the binding energies. Correlate them with the experimental data you will collect or have collected in the lab.
I haven’t been able to find the setting for calculating the vib. freq. of a specific bond. But if we find it, it would be interesting to calculate the vibrational frequency of the metal-oxygen bond for various metal centers.
Add a solvation model, COSMO, to study the effect of a solvent (suggested for Team 5).
Team 5 can also look at the effect of pH by calculating the binding energy with an \(\mathrm{OH^-}\) molecule on the opposite side of the metal center as where the oxygen binds. See https://doi.org/10.1016/j.cattod.2017.05.007 and https://pubs.acs.org/doi/10.1021/jacs.3c11246. However, make sure to have enough time.
Study the effect of geometry optimization on the binding energy. You can try if choosing the LDA XC functional [6] speeds up geometry optimization. Tip: you can make very cool movies if you go to the SCM (top left) -> Movies menu.
Choose a different functional suggested by literature, such as TPSSh [8] or LDA [6], and compare the binding energy.
If you’re curious about something else, feel free to research that instead.
References#
Bin Lv, Xialiang Li, Kai Guo, Jun Ma, Yanzhi Wang, Haitao Lei, Fang Wang, Xiaotong Jin, Qingxin Zhang, Wei Zhang, and others. Controlling oxygen reduction selectivity through steric effects: electrocatalytic two-electron and four-electron oxygen reduction with cobalt porphyrin atropisomers. Angewandte Chemie International Edition, 60(23):12742–12746, 2021. doi:10.1002/anie.202102523.
Mariusz Radon and Kristine Pierloot. Binding of co, no, and o2 to heme by density functional and multireference ab initio calculations. The Journal of Physical Chemistry A, 112(46):11824–11832, 2008. doi:10.1021/jp806075b.
Quan Manh Phung and Kristine Pierloot. The dioxygen adducts of iron and manganese porphyrins: electronic structure and binding energy. Physical Chemistry Chemical Physics, 20(25):17009–17019, 2018. doi:10.1039/C8CP03078B.
Rui Zhang and Jeffrey J Warren. Controlling the oxygen reduction selectivity of asymmetric cobalt porphyrins by using local electrostatic interactions. Journal of the American Chemical Society, 142(31):13426–13434, 2020. doi:10.1021/jacs.0c03861.
Nobuyuki Matsuzawa, Masafumi Ata, and David A Dixon. Density functional theory prediction of the second-order hyperpolarizability of metalloporphines. The Journal of Physical Chemistry, 99(19):7698–7706, 1995. doi:10.1021/j100019a058.
Carme Rovira and Michele Parrinello. Oxygen binding to iron–porphyrin: a density functional study using both lsd and lsd+ gc schemes. International journal of quantum chemistry, 70(2):387–394, 1998. doi:10.1002/(SICI)1097-461X(1998)70:2<387::AID-QUA16>3.0.CO;2-9.
Jhonathan Rosa de Souza, Matheus Morato F de Moraes, Yuri Alexandre Aoto, and Paula Homem-de-Mello. Can one use the electronic absorption spectra of metalloporphyrins to benchmark electronic structure methods? a case study on the cobalt porphyrin. Physical Chemistry Chemical Physics, 22(41):23886–23898, 2020. doi:10.1039/d0cp04699j.
Sebastian Ovalle and Cecile Malardier-Jugroot. Choice of functional for iron porphyrin density functional theory studies: geometry, spin-state, and binding energy analysis. Computational and Theoretical Chemistry, 1213:113726, 2022. doi:10.1016/j.comptc.2022.113726.